Clinical ethnography
Global field research & insight synthesis to inform clinical innovation strategy
Global
2020
This global research initiative was commissioned by a medtech client preparing to launch a novel atrial fibrillation (AFib) ablation technology. The challenge: their breakthrough therapy would first need to be delivered through an existing device with a mixed reputation. The client feared this could erode clinician trust and adoption, so our task was to explore how the product might be received, what would drive confidence, and what future roadmap would support long-term success.
Approach
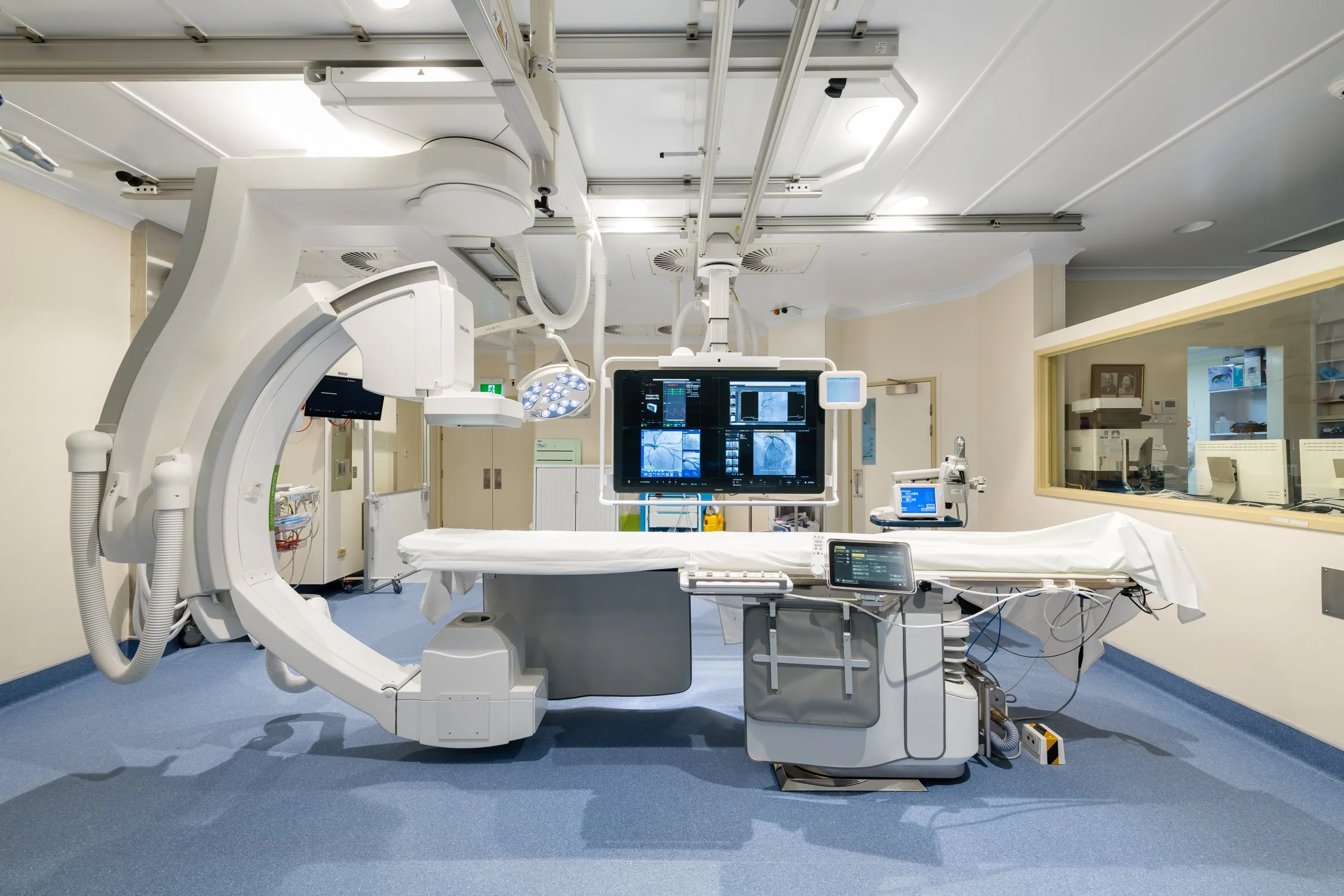
We conducted in-depth ethnographic fieldwork across six countries: Canada, the U.S., Spain, Italy, Hungary, and remote sessions in Japan. Our team observed electrophysiologists inside cath labs, scrubbed in, fly-on-the-wall, documenting not just procedural steps but mental models, workarounds, and decision-making logic in real time.
To support both open insight and directional testing, we included sacrificial design concept stimuli used to elicit visceral feedback and surface implicit preferences. Our methods included:
Observational ethnography
In-depth contextual interviews
Co-analysis with client teams
Insight synthesis into needs, job-to-be-done statements, and strategic implications
Key Insights
The existing delivery device did carry reputational friction—but clinicians were more focused on procedural fit and philosophical alignment
There was no singular workflow: clinicians held widely divergent mental models of atrial fibrillation, shaping how they approached ablation
These models existed on a spectrum from conservative to progressive, and device needs varied significantly across that range
The role of mapping systems was identified as critical—both functionally and symbolically—to support trust in the novel modality
Impact
The research directly influenced two major decisions:
Acquisition: Based on our findings and recommendations, the client made a $1B acquisition of a mapping and navigation company in August 2022, positioning them for stronger procedural integration and clinician buy-in
Successful Launch: The ablation technology launched in December 2023, reframing how ablation is performed globally
Insights were delivered through need statements, strategic frameworks, and an in-person executive workshop with two key audience tiers—guiding both short-term rollout and long-term roadmap planning.